Innate immune signalling and the IRAK pseudokinases
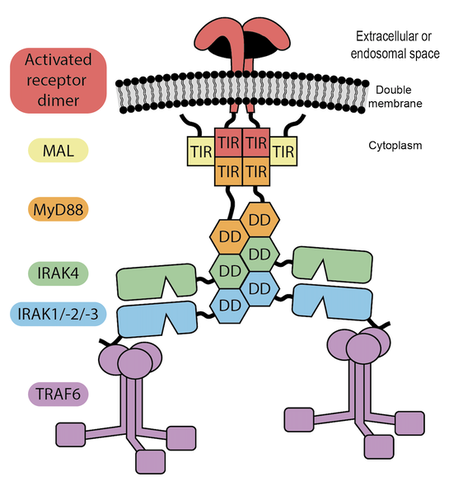
Innate immune responses are the first line of defence that are triggered following the recognition of danger signals by pattern sensing receptors such as the Toll-like receptors (TLRs) and activation of cytokine receptors such as the interleukin-1 receptor (IL-1R). A first step following the activation of these receptors is the assembly of large multi-protein signalling platforms called Myddosomes. Within these platforms, the IL-1R-associated kinases (IRAKs) are modified covalently and are activated to trigger a signalling cascade that culminates in a transcriptional program that shapes the immune response. Despite being the focus of intense study over the past two decades, our understanding of how the IRAK family members are recruited to these complexes and the mechanisms regulating the activity of the IRAKs is still limited. This is because most structural studies to date have investigated components of Myddosomes in isolation. Further, analysis of Myddosomes and innate immune signalling in cells do not provide a clear picture owing to the enormous temporospatial complexities of the system, as well as redundancy and compensatory mechanisms.
Our goal is to use a reconstitution approach to uncover mechanisms of IRAK activation and regulation. We recently solved the crystal structure of the IRAK3 pseudokinase domain, which revealed a novel mechanism by which it may heterodimerize with the autophosphorylated kinase domain of IRAK4. These studies suggest a hypothesis for how this inactive pseudokinase may modulate the activity of IRAK4, a premise we are investigating. Since the Myddosome complex and IRAK family members are at the heart of innate immunity, we anticipate that our research will provide important fundamental insights into how immune responses are activated and reveal how they could be modulated to treat inflammatory diseases such as arthritis, atherosclerosis, systemic lupus erythematosus and psoriasis.
Our goal is to use a reconstitution approach to uncover mechanisms of IRAK activation and regulation. We recently solved the crystal structure of the IRAK3 pseudokinase domain, which revealed a novel mechanism by which it may heterodimerize with the autophosphorylated kinase domain of IRAK4. These studies suggest a hypothesis for how this inactive pseudokinase may modulate the activity of IRAK4, a premise we are investigating. Since the Myddosome complex and IRAK family members are at the heart of innate immunity, we anticipate that our research will provide important fundamental insights into how immune responses are activated and reveal how they could be modulated to treat inflammatory diseases such as arthritis, atherosclerosis, systemic lupus erythematosus and psoriasis.
